If humans are ever to populate Mars, they will need to produce a wide range of organic molecules on the planet, from fuels to pharmaceuticals, that are too expensive to transfer from Earth. Chemists at the University of California, Berkeley, and the Lawrence Berkeley National Laboratory (Berkeley Lab) have devised a strategy to address this.
The researchers have been working on a hybrid system that combines bacteria and nanowires to gather solar energy and convert carbon dioxide and water into building blocks for organic molecules for the past eight years. Nanowires are extremely tiny silicon wires around the diameter of a human hair that are utilized as electrical components, sensors, and solar cells. Peidong Yang, professor of chemistry and director of the S. K. Angela Chan, UC Berkeley's Distinguished Chair in Energy and head of the Kavli Energy Nanoscience Institute, stated: "On Mars, about 96% of the atmosphere is CO2. Basically, all you need is these silicon semiconductor nanowires to take in the solar energy and pass it on to these bugs to do the chemistry for you. For a deep space mission, you care about the payload weight, and biological systems have the advantage that they self-reproduce: You don't need to send a lot. That's why our biohybrid version is highly attractive." The only other requirement, besides sunlight, is water, which on Mars is relatively abundant in the polar ice caps and likely lies frozen underground over most of the planet. |
The biohybrid can also pull carbon dioxide from the atmosphere on Earth to manufacture organic molecules while also addressing climate change, which is caused by an excess of CO2 in the atmosphere produced by humans.
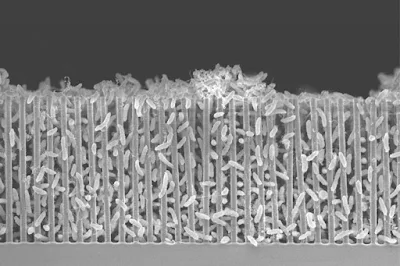
The researchers announce a breakthrough in packing these bacteria (Sporomusa ovata) into a "forest of nanowires" to reach a record efficiency in a new study to be published March 31 in the journal Joule: 3.6 percent of solar energy is transformed and stored in carbon bonds as acetate, a two-carbon molecule that is essentially acetic acid or vinegar.
Acetate molecules may be used to make a variety of organic compounds, such as fuels, polymers, and medicines. Acetate might be used to make a variety of different organic compounds in genetically modified organisms like bacteria and yeast.
Photosynthesis is a natural process in which plants convert carbon dioxide and water into carbon molecules, mostly sugar and carbohydrates. Plants, on the other hand, have a low efficiency, turning less than half of solar energy into carbon molecules. Yang's method is analogous to the sugar cane plant, which converts CO2 to sugar at a rate of 4-5 percent.
Yang is also developing methods to manufacture sugars and carbs from sunshine and CO2, which might provide sustenance for Mars colonists.
Watch the pH
When Yang and his colleagues first demonstrated their nanowire-bacteria hybrid reactor five years ago,the solar conversion efficiency was only approximately 0.4 percent, which is equivalent to plants but still poor when compared to average efficiencies of 20% or higher for silicon solar panels that convert light to electricity. Yang was one of the first 15 years ago to transform nanowires into solar panels.
The researchers subsequently determined that the bugs' production of acetate lowered the acidity of the surrounding water, causing them to detach from the nanowires by increasing pH. He and his students ultimately discovered a means to maintain the water mildly acidic in order to counteract the effect of increasing pH due to constant acetate synthesis. This allowed them to cram a lot more bacteria into the nanowire forest, substantially doubling the efficiency.They were able to keep the reactor running for a week without the bacteria peeling away.
Nanowires were solely employed as conductive wires in this experiment, not as solar absorbers. The electricity was supplied by an external solar panel.
However, in a real-world system, the nanowires would collect light, create electrons, and transfer them to bacteria attached to the nanowires. The bacteria absorb the electrons and transform two carbon dioxide molecules and water into acetate and oxygen, just like plants do.
“These silicon nanowires are essentially like an antenna: They capture the solar photon just like a solar panel,” Yang explained. “Within these silicon nanowires, they will generate electrons and feed them to these bacteria. Then the bacteria absorb CO2, do the chemistry and spit out acetate.”
The oxygen is a side benefit and, on Mars, could replenish colonists’ artificial atmosphere,which would be similar to Earth's 21 percent oxygen environment.
Yang has tinkered with the system in various ways, such as embedding quantum dots in the bacteria's own membrane, which operate as solar panels, collecting sunlight and eliminating the need for silicon nanowires. Acetic acid is also produced by these cyborg bacteria.
His group is still looking for ways to improve the biohybrid's efficiency, as well as approaches for genetically editing bacteria to make them more flexible and capable of creating a wider range of chemical compounds.
The Center for the Utilization of Biological Engineering in Space (CUBES), a multi-university initiative to develop methodologies for biomanufacturing in space, has received funding from NASA.
UC Berkeley co-authors of the paper are current or former graduate students Yude Su, Stefano Cestellos-Blanco and Ji Min Kim, who contributed equally to the work; and graduate students Yue-xiao Shen, Qiao Kong, Dylan Lu, Chong Liu, Hao Zhang and Yuhong Cao.